
Euchromatin is the lightly packed form of chromatin, whereas heterochromatin refers to the condensed form.
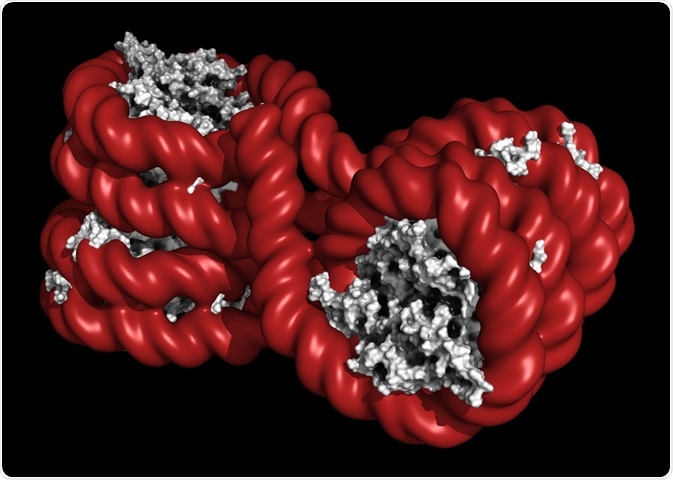 Image Credit: molekuul_be / Shutterstock
Image Credit: molekuul_be / Shutterstock
Euchromatin and heterochromatin are functionally and structurally distinct, and have key roles in the transcription and expression of genes.
Chromatin
A chromatin is comprised of 147 base pairs of DNA wrapped around a protein core referred to as a histone.
The histone is comprised of two units each of H2A, H2B, H3, and H4, making an octamer.
The organization of the chromatin is not the same throughout the whole genome, leading to the formation of more condensed regions and less condensed regions.
Heterochromatin
Structure of heterochromatin
Heterochromatin is very tightly packed and condensed. The formation of heterochromatin involves modifications to histones and the recruitment and spreading of silencing complexes, which cause changes to the structure of chromatin. These changes affect various DNA processes, including gene transcription.
Heterochromatin is inhibitory to the expression of genes within it, due to its repressive structure. Heterochromatin can fold into higher order structures, and its formation induces an increase in negative supercoiling of DNA.
Heterochromatin is very stable in structure but is also dynamic and changes with the cell cycle.
Within heterochromatin, there are DNA elements called barriers that promote the formation of active chromatin and the exclusion of nucleosomes, this allows heterochromatin to spread.
Functions of heterochromatin
There are many chromatin modifications that can be used to identify heterochromatin function.
Within yeast, heterochromatin core histones are hypoacetylated. This makes the lysine residues more positively charged, which increases the interaction between the histone and the DNA, making the nucleosome more closed in structure.
Histone H4-K16 is less acetylated in heterochromatin, which promotes the folding of the chromatin into high structure orders, further promoting a closed chromatin structure.
Heterochromatin is also hypomethylated at H3-K4 and K79, which indicates that the heterochromatin is less transcriptionally active.
Silencing (SIR) complexes are comprised of Sir2, Sir3, and Sir4 proteins that associate with each other to form a complex.
The SIR complex plays a role in the formation of heterochromatin. Sir2 is a NAD-dependent protein deacetylase that interacts with Sir4 and deacetylases lysine in histones.
Sir3 also acts at various points in the chromatin to induce chromatin silencing. Sir4 induces the formation of the SIR complex which leads to further silencing of chromatins.
DNA within heterochromatin is less accessible than euchromatin due to the presence of the aforementioned chromatin modifications.
Euchromatin
Structure of euchromatin
Euchromatin consists of loosely wrapped chromatin, so the DNA is more accessible. Modifications to the histone tails allow the chromatin to be more open.
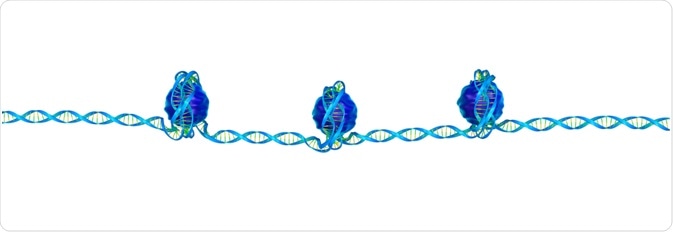 Image Credit: sciencepics / Shutterstock
Image Credit: sciencepics / Shutterstock
As discussed above, the modifications that cause heterochromatin to be closed off to DNA replication enzymes are reversed in euchromatin.
Functions of euchromatin
Euchromatin is the part of the chromatin involved in the active transcription of DNA into mRNA. As euchromatin is more open in order to allow the recruitment of RNA polymerase complexes and gene regulatory proteins, so transcription can be initiated.
There is a direct link between how actively productive a cell is and the amount of euchromatin in its nucleus.
Euchromatin to heterochromatin transition
Euchromatin can be transitioned into heterochromatin, which can control gene expression within a cell. Processes, such as the cell cycle, use this to regulate the transcription of different genes throughout the cell cycle.
The transition can occur in other events, such as during an infection.
Kaposi’s sarcoma-associated herpesvirus (KSHV) can affect chromatin, which promotes latent gene expression but suppresses lytic gene expression. Initially, the KSHV contains euchromatin, with high levels of H3K4me3 and H3K27ac.
After de novo infection, the levels of those histones decline on the KSHV genome and the levels of the repressive histones, H3K27me and H2AK119ub,increase.
This indicates the transition from euchromatin to heterochromatin within the KSHV genome.
This transition is also dependent on Polycomb repressive complex 1 and 2. When the KSHV encounters epithelial cells, it adopts more heterochromatin, which results in a latent infection.
However, when a virus encounters oral epithelial cells, it adopts more euchromatin, which results in lytic gene expression that facilitates viral replication.
In conclusion, euchromatin and heterochromatin are both needed for control processes within DNA replication.
The structure of the chromatin is heavily tied to its function, and euchromatin and heterochromatin can change depending on the requirements of the genes within them.
It has also been demonstrated that viruses can manipulate euchromatin/heterochromatin within their genomes to achieve certain tasks.
Sources:
- https://iubmb.onlinelibrary.wiley.com/doi/full/10.1002/iub.1322
- https://www.ncbi.nlm.nih.gov/pubmed/24367262
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3868514/
Further Reading
- All Epigenetics Content
- Epigenetics and Epigenomics
- What is Epigenomics?
- Epigenetics and Forensics
- Epigenetics for Multiple Sclerosis
Last Updated: Aug 30, 2018
Written by
Samuel Mckenzie
Sam graduated from the University of Manchester with a B.Sc. (Hons) in Biomedical Sciences. He has experience in a wide range of life science topics, including; Biochemistry, Molecular Biology, Anatomy and Physiology, Developmental Biology, Cell Biology, Immunology, Neurology and Genetics.
Source: Read Full Article