
A case report describes a novel helmet device that generates a noninvasive oscillating magnetic field and that shrunk a glioblastoma tumor by about a third.
This is the first time that the wearable Oncomagnetic device was tried with a patient.
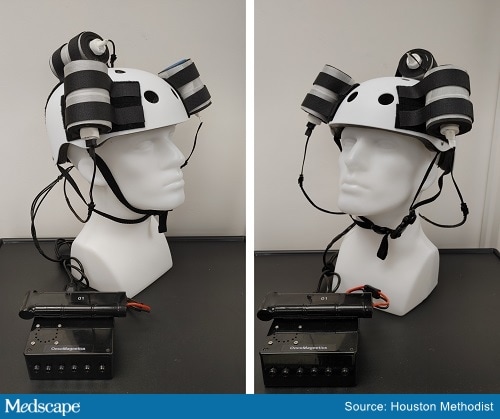
The Oncomagnetic device.
The patient had end-stage recurrent glioblastoma and had undergone all standard therapy options. He wore the device for 5 weeks but died from an unrelated injury, so the treatment period was cut short.
A brain scan showed a 31% reduction of contrast-enhanced tumor volume, and an autopsy of his brain confirmed the rapid response to the treatment.
The case study was published online on July 22 in Frontiers in Oncology.
“I believe that there is a great potential with this device,” said study author David S. Baskin, MD, director of the Kenneth R. Peak Center for Brain and Pituitary Tumor Treatment in the Department of Neurosurgery at Houston Methodist Hospital, Houston, Texas. “This is a very exciting time.”
Baskin and two co-authors are listed as co-inventors of the device on a US patent application filed by Houston Methodist Hospital.
The team is now treating several patients with glioblastoma under compassionate use.
Approached by Medscape Medical News for an independent comment, Adilia Hormigo, MD, PhD, director of the Neuro-Oncology Program at the Tisch Cancer Institute, Mount Sinai Health System, New York City, noted that a clinical trial is needed to evaluate the device. “But this is an interesting idea, and we have to be open-minded in treating this fatal disease,” she said.
Oscillating Magnetic Fields
The Oncomagnetic device consists of three oncoscillators that are attached to the outside of a helmet and are connected to a microprocessor-based electronic controller powered by a rechargeable battery.
It consists of a series of rotating magnets that produce oscillating magnetic fields that cover the entire brain, including the upper part of the brain stem. The device induces rapid apoptosis of glioblastoma cells, Baskin explained. Its mechanism of action involves disruption of the electron transport in the mitochondrial respiratory chain, causing an elevation of reactive oxygen species and caspase-dependent cancer cell death.
Baskin emphasized that the new Oncomagnetic device is very different from the Optune device (Novocare), which is already approved by the US Food and Drug Administration (FDA) and has been shown to increase survival among patients with glioblastoma. Optune uses tumor-treating fields (TTFs), which are electromagnetic waves that are delivered via an electric field generator through four transducer arrays that are placed on a shaved scalp. Preclinical studies indicated that the TTFs disrupt cell division by disrupting several steps in the mitotic process that are crucial for cell division.
Both of these devices “are using a type of external maneuver” rather than invasive intracranial approaches, said Hormingo. The experimental Oncomagnetic device may have an advantage in that it needs to be worn by the patient for fewer hours, she commented. A better understanding of the physics and underlying mechanism is needed, however. Clinical trials are an essential next step, she emphasized.
Most Common Brain Cancer in Adults
Glioblastoma is the most common malignant tumor of the brain in adults. Outcomes continue to be dismal. In more than 40 years, median survival has only modestly improved.
“We haven’t gotten very far with glioblastoma despite millions of dollars in research,” Baskin said. “With treatment, survival is about 15 months, and those are not very good months.”
Out of the Box
Standard treatments for glioblastoma include surgery, radiotherapy, and chemotherapy, and many patients cannot tolerate some of these, Baskin noted. Hence, there is a great need for a different therapeutic approach that yields better outcomes with lower toxicity.
“We didn’t want to develop another chemotherapeutic agent that would help you live another 2 months,” he told Medscape Medical News. “We were trying to think out of the box.
“If you want to do something that will really make a difference in an aggressive tumor like glioblastoma, you have to attack something so basic that the tumor can’t evade it,” he said. “For example, with temozolomide, if it is unmethylated, the tumor can repair the DNA damage from the chemotherapy. Even if you’re sensitive to begin with, over time, the tumor will eventually become resistant.”
The new device stems from work by Baskin and colleagues on mitochondria, which he describes as the powerhouse of the cell. “Mitochondrial DNA can’t repair itself, so if you damage the mitrochondria, you will damage the cell, and theoretically, it cannot repair itself,” he said.
In preclinical models, the oscillating magnetic fields generated by the new device were shown to kill patient-derived glioblastoma cells in cell culture without having cytotoxic effects on cortical neurons and normal human astrocytes. Animal studies also showed that it was effective and nontoxic, explained Baskin.
However, getting the device to human clinical trials has been slow going. “We wanted to start an early-phase trial for an investigational device, but the FDA is overwhelmed with COVID-related applications,” he said. “That has taken priority, and we understand that. So we were able to evaluate it on a patient through compassionate use via the FDA-approved Expanded Access Program.”
Exciting Possibilities
The patient was a 53-year-old man who had undergone radiotherapy and chemotherapy, and the tumor was progressing. Imaging revealed the presence of leptomeningeal disease, which is associated with a poor outcome and a median survival of 3.5 to 3.9 months.
The patient was fitted with the helmet device and wore it under supervision for the first 3 days of treatment, during which time the strength of the oscillating magnetic fields was escalated. After this initial supervised phase, the treatment continued at home without supervision, using the same regimen as on the third day.
Treatment was first administered for 2 hours while under supervision and was then gradually increased to a maximum of 6 h/d. The patient was evaluated clinically on days 7, 16, 30, and 44 after initiation of treatment. No serious adverse events were reported during treatment. The patient’s wife reported subjective improvement in speech and cognitive function.
Baskin noted that the patient had been experiencing falls for the past year and a half before treatment was initiated. “And then he tripped and fell and sustained a head injury that he subsequently died from,” he said.
Autopsy results confirmed the rapid response to treatment, and tumor shrinkage appeared to correlate with the treatment dose.
“Our results in the laboratory and with this patient open a new world of noninvasive and nontoxic therapy for brain cancer, with many exciting possibilities for the future,” Baskin commented.
He said his team has experimented with this approach with other tumor types in the laboratory, including triple-negative breast cancer and lung cancer. “We’ve only tried it in a culture so far, but it seems to melt the cancer cells,” he said.
The work was supported by a grant from the Translational Research Initiative of the Houston Methodist Research Institute and several Foundations. Baskin and two co-authors are listed as inventors on a US patent application filed by Houston Methodist Hospital for the device used in this report.
Front Oncol. 22 July 2021. Full text
For more from Medscape Oncology, join us on Twitter and Facebook.
Source: Read Full Article