
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pathogenesis is a multistep process that involves the infection of angiotensin-converting enzyme 2 (ACE2)-expressing lung epithelial cells followed by uncontrolled viral replication, which leads to cell lysis and the release of damage-associated molecular patterns (DAMPs).
When the neighboring cells recognize these molecules, they produce a pro-inflammatory environment by releasing cytokines such as Interleukin 6 (IL-6) and Interleukin-10 (IL-10), which recruit and activate macrophages, monocytes, and T cells (also called T lymphocytes).
.jpg)
In the case of severe COVID-19 patients, this pro-inflammatory feedback loop leads to a harmful response that causes structural damage to the lung. The cytokine storm that results can cause acute respiratory distress syndrome (ARDS) and organ failure. Although DAMPs can drive systemic inflammation in many settings, it is unclear if it occurs in COVID-19.
“Aberrant and rapid increase in a broad-spectrum of pro-inflammatory cytokines, known as a cytokine storm, plays a central role in the pathogenesis of ARDS and other severe complications of SARS-CoV-2 infection.”
Evaluating the impact of CD24Fc treatment in severe COVID-19 patients
The therapeutic efficacy of anti-viral and anti-inflammatory agents against SARS-CoV-2 has been shown to be limited. Soluble CD24 (CD24Fc), a highly glycosylated protein, has been shown to diminish the overall inflammatory response induced by DAMPs. A recent randomized phase III trial conducted by researchers at the Ohio State University, Columbus, OH, US, evaluated the impact of CD24Fc in severe COVID-19 patients and showed promising clinical efficacy. This study is available as a preprint on the medRxiv* server.
The researchers analyzed peripheral blood samples collected from 22 patients enrolled in the SAC-COVID trial (NCT04317040). The samples were collected before and at many points after CD24Fc or placebo treatment. They performed high-dimensional spectral flow cytometry analysis and assessed cytokine levels to determine the immunological impact of treatment with CD24Fc on COVID-19 patients.
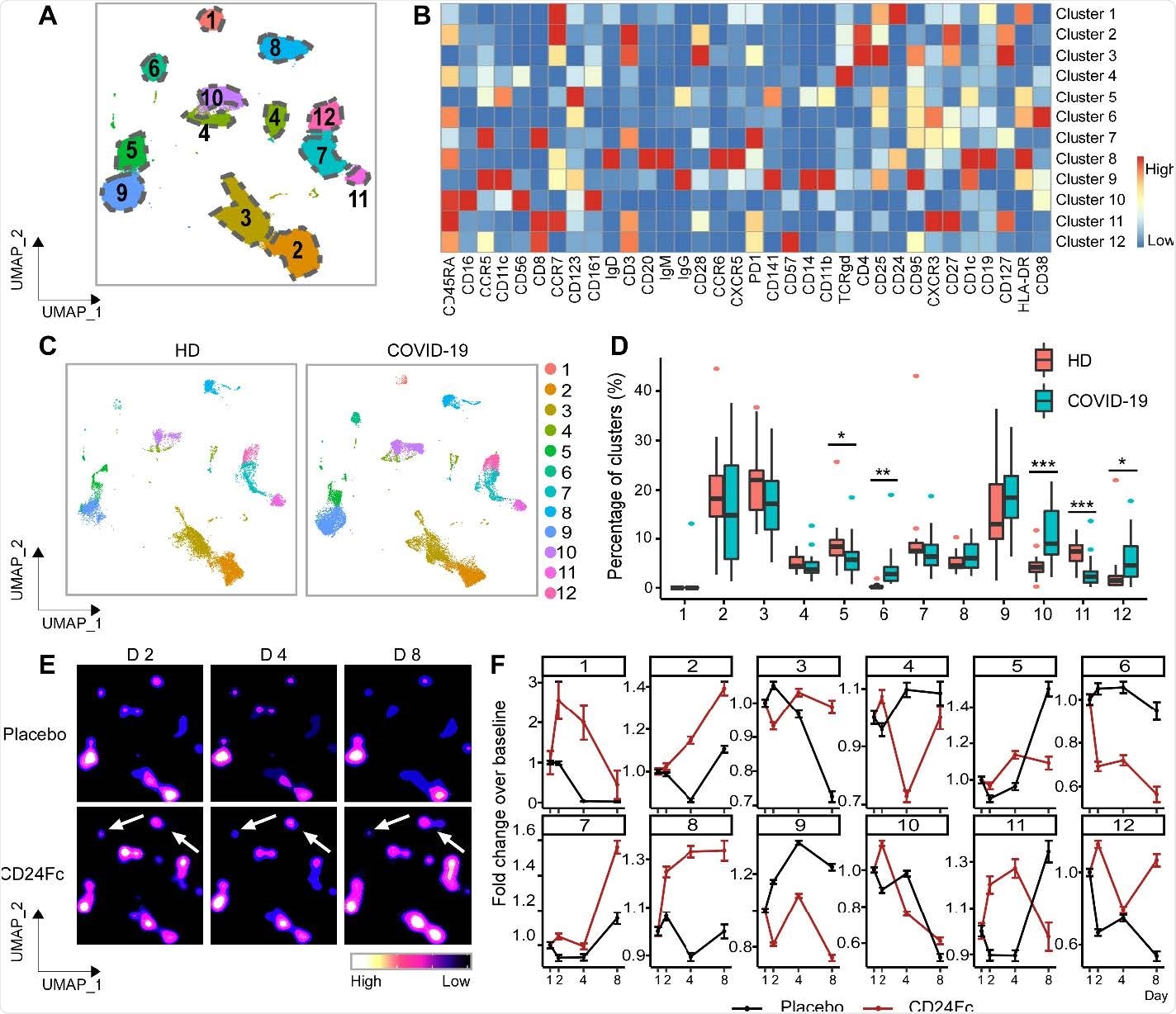
Patient characteristics from the CD24Fc vs. placebo groups were matched clinically so as to compare results without confounding factors. With the help of high-content spectral flow cytometry, the researchers found systemic hyper-activation of multiple cell compartments in the placebo group, including CD4+ T cells, CD8+ T cells, and CD56+ NK cells in patients having untreated COVID-19.
“In addition to the two therapeutic targets, we also identified two cytokines that were significantly downregulated after CD24Fc treatment: IL-10 and IL-15.”
In comparison, CD24Fc-treated patient samples showed dampened systemic inflammation, with a return to homeostasis in NK and T cells within days. In addition, a single CD24Fc dose significantly weakened systemic IL-10 and IL-15 cytokines and dampened the coexpression and networking among inflammatory cytokines associated with severe COVID-19.
“Our clinical and immunological data support further development of CD24Fc as a novel therapeutic against severe COVID-19.”
CD24Fc treatment leads to accelerated recovery in severe COVID-19 patients compared to placebo treatment
The severe COVID-19 patients in this study showed accelerated recovery after CD24Fc treatment compared to placebo treatment. CD24Fc was mostly well tolerated, decreased disease progression, and shortened length of hospital stay in pts with COVID-19. Based on SARS-CoV-2’s proposed mechanism of action and pathophysiology, the authors hypothesized that CD24Fc decreased the hyperactive systemic immune responses in patients which led to an accelerated return to homeostasis.
In conclusion, the study results offer unique insights into the immunological impact of SARS-CoV-2 that complement the clinical findings of the SAC-COVID trial. Furthermore, these findings strongly support further investigation of CD24Fc for many inflammatory conditions, including COVID-19.
“Our unique cytokine centrality analysis and cellular activation index also warrant further study as a prognostic tool for guiding therapy in COVID-19 and other systemic inflammatory conditions.”
*Important Notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Immunological Insights Into the Therapeutic Roles of CD24Fc Against Severe COVID-19, No-Joon Song, Carter Allen, Anna E. Vilgelm, Brian P. Riesenberg, Kevin P. Weller, Kelsi Reynolds, Karthik B. Chakravarthy, Amrendra Kumar, Aastha Khatiwada, Zequn Sun, Anjun Ma, Yuzhou Chang, Mohamed Yusuf, Anqi Li, Cong Zeng, John P. Evans, Donna Bucci, Manuja Gunasena, Menglin Xu, Namal P.M. Liyanage, Chelsea Bolyard, Maria Velegraki, Shan-Lu Liu, Qin Ma, Martin Devenport, Yang Liu, Pan Zheng, Carlos D. Malvestutto, Dongjun Chung, Zihai Li, medRxiv, 2021.08.18.21262258; doi: https://doi.org/10.1101/2021.08.18.21262258, https://www.medrxiv.org/content/10.1101/2021.08.18.21262258v1
Posted in: Drug Trial News | Medical Research News | Disease/Infection News | Pharmaceutical News
Tags: ACE2, Acute Respiratory Distress Syndrome, Angiotensin, Angiotensin-Converting Enzyme 2, Anti-Inflammatory, Blood, CD4, Cell, Cell Lysis, Coronavirus, Coronavirus Disease COVID-19, Cytokine, Cytokines, Cytometry, Efficacy, Enzyme, Flow Cytometry, Hospital, Inflammation, Pathophysiology, Placebo, Protein, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome

Written by
Susha Cheriyedath
Susha has a Bachelor of Science (B.Sc.) degree in Chemistry and Master of Science (M.Sc) degree in Biochemistry from the University of Calicut, India. She always had a keen interest in medical and health science. As part of her masters degree, she specialized in Biochemistry, with an emphasis on Microbiology, Physiology, Biotechnology, and Nutrition. In her spare time, she loves to cook up a storm in the kitchen with her super-messy baking experiments.
Source: Read Full Article