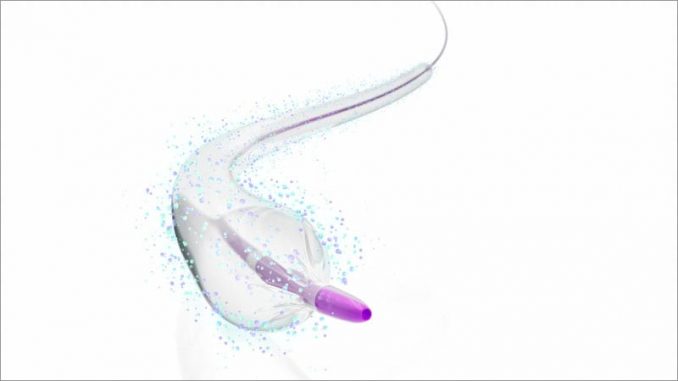
The US Food and Drug Administration has approved the IN.PACT 018 drug-coated balloon (DCB) for the treatment of peripheral arterial disease, Medtronic announced today.
The paclitaxel-coated balloon is indicated for percutaneous transluminal angioplasty of de novo, restenotic, or in-stent restenotic lesions up to 360 mm in length with vessel diameters of 4 to 7 mm, located in the superficial femoral and popliteal arteries.
The IN.PACT 018 DCB is built on the same technology as the IN.PACT Admiral DCB (0.035 inch guidewire compatible), but is engineered to cross tight lesions, provide better deliverability, and is 0.018 inch guidewire compatible, according to a company news release.
“The IN.PACT 018 DCB will be the only commercially available DCB with a 200 cm over-the-wire (OTW) catheter length, providing physicians the option to treat via femoral or radial access,” Medtronic stated. This is in addition to the 130 cm OTW catheter length.
The device has not been evaluated in a clinical study, but its approval was supported by clinical studies, performed primarily via femoral access, establishing the safety and effectiveness of the IN.PACT Admiral DCB.
Follow Patrice Wendling on Twitter: @pwendl For more from theheart.org | Medscape Cardiology, follow us on Twitter and Facebook.
Source: Read Full Article