
The enzyme caspase-8 induces a molecular cell death programme called pyroptosis without involving its enzymatic activity, a new study by Hamid Kashkar published in Nature shows. In order to safeguard healthy and functioning tissues, cells utilize different cell death mechanisms to dispose of unwanted cells (e.g. infected or aged cells). Apoptosis is a ‘cellular suicide programme’ that does not cause tissue injury and is induced by caspase-8.
Necroptosis is another mode of regulated cell death which causes cellular damage and is normally engaged when caspase-8 is inhibited. Pyroptosis describes an inflammatory mode of regulated cellular death process, which is normally activated in response to microbial pathogens and is central for mounting anti-microbial immunity. Hamid Kashkar and his team have now shown that caspase-8 not only controls apoptosis and necroptosis but pyroptosis as well. The study “Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis” was published in Nature.
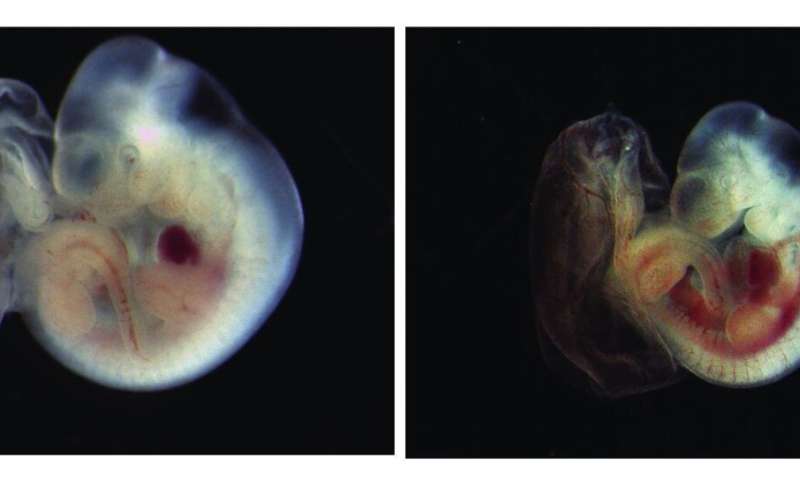
The research team studied the biological roles of caspase-8 in cell cultures and mice. Kashkar’s group showed that the enzymatic activity of caspase-8 is required to inhibit pyroptosis. “We found out that the expression of inactive caspase-8 causes embryonic lethality and inflammatory tissue destruction. This could only be restored when necroptosis and pyroptosis were simultaneously blocked,” Hamid Kashkar explains. The lack of caspase-8 enzymatic activity primarily causes necroptotic cell death. Interestingly, when necroptosis is blocked, the inactive caspase-8 serves as a protein scaffold for the formation of a signalling protein complex called inflammasome, which ultimately induces pyroptosis. “Microbial pathogens are heavily reliant on the fate of infected cells and have evolved a number of strategies to inhibit apoptosis and necroptosis,” Hamid Kashkar adds.
Source: Read Full Article