Scientists have found an experimental small molecule that induces a form of cell death to kill a variety of cancers while enhancing the power of the immune system and leaving healthy cells totally unscathed.
The molecule triggers ferroptosis, a unique form of cell death that is increasingly being tested as an anti-cancer strategy. The international team of scientists have reported their findings in the journal Science Translational Medicine.
For students of biology, the three major forms of cell death are well known and taught early in undergraduate general biology courses. They are apoptosis, or type 1 cell death; autophagy, type 2 cell death; and cell necrosis, or type 3. Ferroptosis, by contrast, is a distinct form of cell death that relies on a buildup of iron and the generation of reactive oxygen species, which ultimately cause a doomed cell to self-destruct.
Turning to a different form of cell death is vital because most forms of cancer treatment today trigger cell death by enzyme-dependent apoptosis. Unfortunately, a key hallmark of human cancers is their capacity to develop resistance to treatment, and many tumor types have shockingly developed resistance to apoptosis, hence the hunt for a different way to kill tumor cells.
As intriguing as ferroptosis may seem as an alternative, it is not yet ready to be exploited as a formal therapeutic to treat cancers in people. But the fact that it isn’t yet ready for prime time doesn’t mean that it won’t be in the not-too-distant future.
“We expect that such an approach warrants further preclinical evaluation for its possible incorporation into the oncological armamentarium,” writes lead author Dr. Jingbo Li, a researcher in the Department of Surgery at the University of Texas Southwestern Medical Center in Dallas, Texas. Li is also affiliated with the Department of Gastroenterology in Xiangya Hospital of Central South University in Changsha, China.
Even though ferroptosis is being actively pursued as a potential cancer treatment, other researchers are studying the process for its pathological role in a variety of disparate diseases that range from Alzheimer’s, cardiovascular disease to even various forms of cancer. Ferroptosis is intimately involved in the disease processes of these conditions, studies have shown.
In terms of harnessing this form of cell death as a therapeutic, teams worldwide are racing to overcome a number of conundrums, especially what’s known as the “non-selective” activities of ferroptotic cell death. It doesn’t just kill cancer cells, it kills a multitude of cells in the immediate micro-environment, particularly the Big Three: dendritic cells, T cells and neutrophils, which seemingly defeats the promise that ferroptosis holds as a cancer fighter.
That means most compounds capable of inducing ferroptosis in cancer cells may also inflict the same fate on various immune cells, weakening the immune system’s ability to swoop in and wage war on deadly tumors.
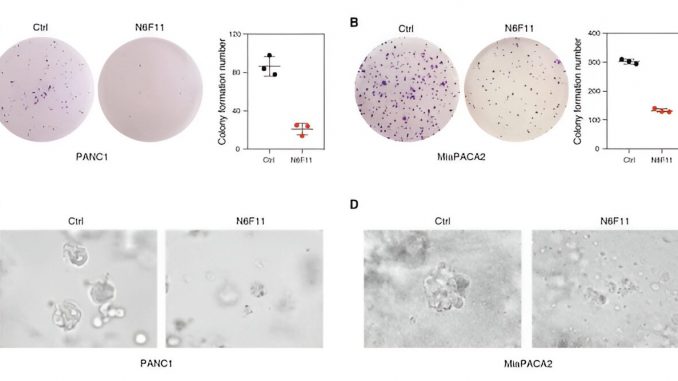
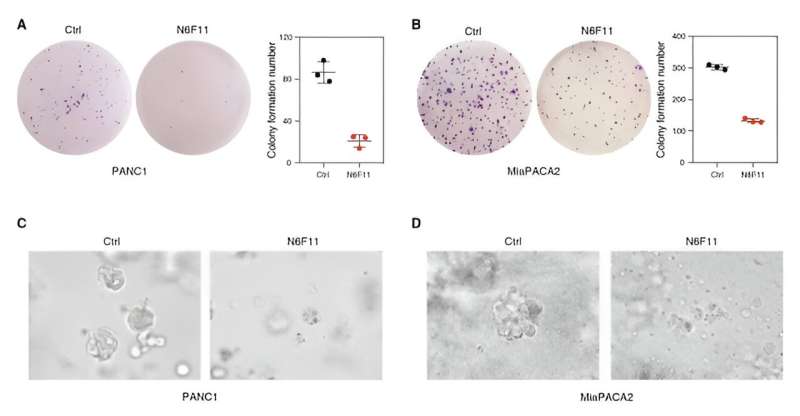
Now, Li and a far-flung team of collaborators on three continents have pinpointed a promising small molecule called N6F11, which not only triggers ferroptotic cell death, it also selectively causes the degradation of glutathione peroxidase-4, also known as GPX4, a notorious blocker of ferroptosis.
Li and colleagues, who went on a wide-ranging drug hunt, screening a slew of compounds to find N6F11 and its unique properties, say with N6F11 in the mix, ferroptosis can be triggered and GPX4 is no longer in the way to prevent this specialized form of cell death from annihilating tumors. Even more eye-opening, N6F11 degraded GPX4 in human pancreatic, bladder, breast, and cervical cancer cells without affecting GPX4 in that vital trio of the immune system: dendritic cells, T cells and neutrophils.
Li, along with colleagues from Columbia University in New York, Université de Paris in France, a large team at UT Southwestern Medical Center in Dallas and beyond, also found that N6F11 slowed the growth of active tumors in mouse models inoculated with pancreatic cancer cells. The animals endured the treatment without severe side effects—an effect the authors tied to N6F11’s ability to stimulate T cells.
“Lipid peroxidation-dependent ferroptosis has become an emerging strategy for tumor therapy,” Li added. “However, current strategies not only selectively induce ferroptosis in malignant cells but also trigger ferroptosis in immune cells simultaneously, which can compromise anti-tumor immunity,”
Li explained in Science Translational Medicine that by using In-Cell Western assays, combined with the massive drug screening, the team was able to identify and confirm the compound N6F11 as a ferroptosis inducer.
“An effective antitumor adaptive immune response requires dendritic cells that present tumor-associated antigens to T lymphocytes,” Li continued. “The initiation of the anticancer response by dendritic cells largely depends on sufficient immune signals, particularly pathogen-associated molecular patterns and damage-associated molecular patterns.
“The induction of immunogenic cell death by chemotherapy, radiotherapy, or targeted therapies can initiate an adaptive immune response by exposing and releasing a variety of damage-associated molecular patterns.
“However, it is not known which type of cell death optimally enhances the immunogenicity of tumor cells,” Li added, noting that ferroptotic cell death depends on unrestricted lipid peroxidation rather the cascade of enzymatic events associated with apoptosis.
“In summary, we identified a small molecule, N6F11, that induces the selective degradation of GPX4 in malignant, but not immune, cells,” Li concluded. “In small tumors, N6F11-induced ferroptosis [and] initiates a powerful antitumor immune system.”
More information:
Jingbo Li et al, Tumor-specific GPX4 degradation enhances ferroptosis-initiated antitumor immune response in mouse models of pancreatic cancer, Science Translational Medicine (2023). DOI: 10.1126/scitranslmed.adg3049
Journal information:
Science Translational Medicine
© 2023 Science X Network
Source: Read Full Article