
On April 15, 2020, research group from Osaka City University (OCU) launched a clinical trial aiming at investigating the performance and clinical efficacy of a newly developed COVID-19 antibody detection kit. The study will be led by Associate Professor Yasutoshi Kido, Lecturer Yu Nakagama and Professor Akira Kaneko from the Department of Parasitology, as well as Professor Hiroshi Kakeya from the Department of Infection Control Science and Professor Yasumitsu Mizobata from the Department of Traumatology and Critical Care Medicine, using a testing platform developed by a collaboration with Mokobio Biotechnology R & D Center Inc.
The current, most widely used method for COVID-19 testing is based on PCR technology. However, PCR-based diagnosis still harbors issues to be solved, regarding accuracy, procedural simplicity, and cost-effectiveness. The high prevalence of mildly symptomatic or even asymptomatic patients causing the explosive spread of COVID-19, calls for an alternative to PCR-based testing in order for us to cope against this emerging infectious disease. For this urgent need to compensate the limitations of PCR-based diagnosis, the research group proposes a novel method which monitors the intensity of the host immune response, by measuring human serum antibody titer specific to the SARS-CoV2 virus.
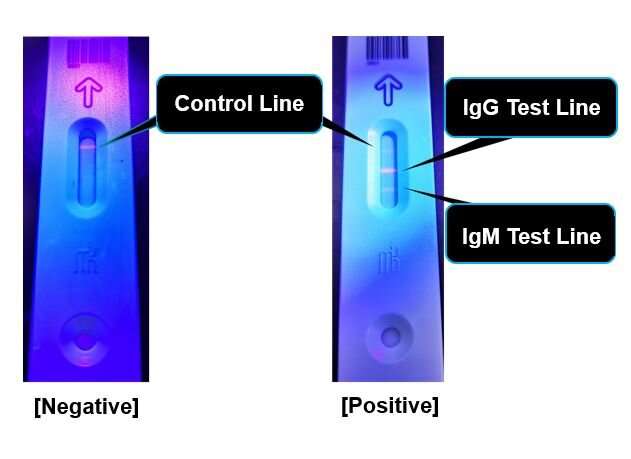
The human host immune cells are capable of producing “antibodies” which specifically capture proteins originating from infectious viruses. If not infected with the COVID-19 causing SARS-CoV2, antibodies against SARS-CoV2 derived proteins will be absent from blood. The IgM antibody usually appears during the early stage of infection and the IgG antibody will follow as the illness shifts towards the recovery stage. Testing for various COVID-19 antibodies has the potential to efficiently diagnose and further estimate the stage of disease.
By its unique fluorescence-based semi-quantitative detection system, the novel method avoids interobserver judgment errors by digitalizing the measurement process. Amount of blood needed for testing is as small as 20 μL. Avoiding the need to obtain nasopharyngeal swab specimens will reduce the risk of infection among medical personnel who are responsible for patients’ sample collection.
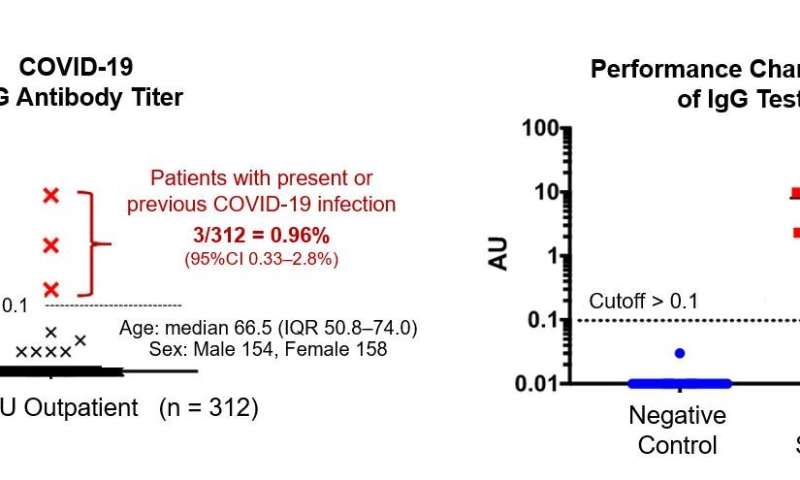
In their pilot trial, the researchers randomly sampled residual serum specimens from the OCU outpatient clinic and investigated the rate of positivity for the COVID-19 IgG antibody. Among 312 participants who all had visited the hospital for non-COVID-19 purposes, the anti-SARS-CoV2 IgG antibody was present in three, representing up to approximately 1% (median 0.96, IQR 0.33-2.8%). Although limitations exist in extrapolating the finding to the IgG prevalence among healthy Osaka citizens, they state that the reported number of PCR-confirmed symptomatic cases may be only representing the tip of an iceberg of the whole burden of infection.
Source: Read Full Article