
While carcinogens are widely known to be perpetrators of cancer, a new study from researchers at the Mass General Cancer Center has shown that exposure to carcinogens can increase cancer cells’ susceptibility to immune attack.
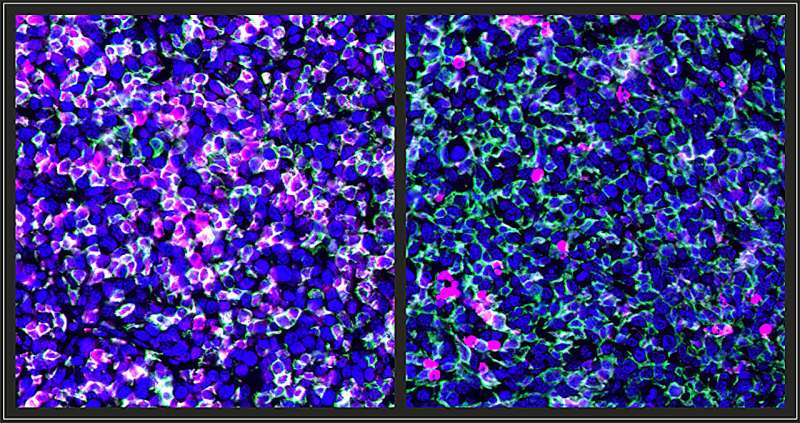
Cancerous cells create tumor microenvironments (TME) in which they take over healthy immune cells and promote cancer growth. The researchers hypothesized that DNA-mutating carcinogens may enhance the immune response to cancer. In their study, they tested breast cancer cells from mice and lung cancer cells from humans and found that these cells, when exposed to carcinogens, were unable to create an immunosuppressive TME, which boosts the immune response to reject the cancer.
The results, published in the Journal of Clinical Investigation, showed that carcinogens block immunosuppressive TMEs, which induced tumor-associated macrophages (TAMs) that have antitumor properties. The discovered pathway points to immune factors that could be targeted to increase the effectiveness of immunotherapy treatments for cancer patients.
“Our findings support the notion that carcinogen exposure not only enhances T cell immunity by increasing tumor antigens, but also alters TAM differentiation, which enhances tumor immunogenicity,” said Shawn Demehri, MD, Ph.D., principal investigator at the Center for Cancer Immunology and Cutaneous Biology Research Center and a dermatologist at Massachusetts General Hospital.
“Although we have discovered the first immune-inducing pathway downstream of carcinogen exposure that is antigen independent, we aim to explore other pathways that may also contribute to the immunogenic transformation of cancers by carcinogens. These pathways can then be exploited to further improve cancer immunotherapy.”
More information:
Mei Huang et al, Carcinogen exposure enhances cancer immunogenicity by blocking the development of an immunosuppressive tumor microenvironment, Journal of Clinical Investigation (2023). DOI: 10.1172/JCI166494
Journal information:
Journal of Clinical Investigation
Source: Read Full Article