BRETARIS® GENUAIR®
aclidinium bromide
Consumer Medicine Information (CMI)
What is in this leaflet
This leaflet answers some common questions about BRETARIS GENUAIR.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your pharmacist or doctor will be able to advise you about the risks and benefits of using BRETARIS GENUAIR.
If you have any concerns about using this medicine, ask your pharmacist or doctor.
Keep this leaflet with the medicine.
You may need to read it again.
What BRETARIS GENUAIR is used for
The active ingredient of BRETARIS GENUAIR is aclidinium bromide, which belongs to a group of medicines called bronchodilators. Bronchodilators relax airways and help keep bronchioles open.
BRETARIS GENUAIR is a dry powder inhaler that uses your breath to deliver the medicine directly into your lungs. This makes it easier for chronic obstructive pulmonary disease (COPD) patients to breathe.
BRETARIS GENUAIR is indicated to help open the airways and relieve symptoms of COPD, a serious, long-term lung disease characterised by breathing difficulties. Regular use of BRETARIS GENUAIR can help you when you have ongoing shortness of breath related to your disease and will help you to minimise the effects of the disease on your everyday life.
BRETARIS GENUAIR is indicated for maintenance treatment of your COPD; it should not be used to treat a sudden attack of breathlessness or wheezing. If your COPD symptoms (breathlessness, wheezing, cough) do not improve or get worse you should contact your doctor for advice as soon as possible.
Ask your doctor if you have any questions about why BRETARIS GENUAIR has been prescribed for you.
This medicine is only available with a doctor’s prescription.
It is not addictive.
Before you use BRETARIS GENUAIR
When you must not use it
Do not use BRETARIS GENUAIR if you have an allergy to:
aclidinium bromide or any of the other ingredients of this medicine listed at the end of this leaflet
atropine or related bronchodilator medicines, for example ipratropium, tiotropium or oxitropium.
Some of the symptoms of an allergic reaction may include:
shortness of breath
wheezing or difficulty breathing
swelling of the face, lips, tongue or other parts of the body
rash, itching or hives on the skin.
Do not use this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering.
In that case, return it to your pharmacist.
Before you start to use it
Tell your doctor if you have any of the following medical conditions:
if you have asthma
if you have had heart problems recently
if you see halos around lights and coloured images, have eye pain or discomfort or temporary blurring of vision (narrow angle glaucoma)
if you have an enlarged prostate, problems passing urine, or a blockage in your bladder
if you have rare hereditary galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption.
Your doctor may want to take special precautions if you have any of the above conditions.
BRETARIS GENUAIR may cause dryness in the mouth, which may cause tooth decay after using your medicine for a long time. Therefore, please remember to pay attention to oral hygiene.
Tell your doctor if you are pregnant, intend to become pregnant or are breastfeeding.
BRETARIS GENUAIR is not recommended for use during pregnancy or while breastfeeding. If it is necessary for you to use this medicine during pregnancy or while breastfeeding, your doctor will discuss with you the benefits and risks involved.
Children and adolescents
BRETARIS GENUAIR is not for use in children or adolescents below 18 years of age.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
Some medicines and BRETARIS GENUAIR may interfere with each other. These include other medicines used to treat breathing difficulties, such as tiotropium, ipratropium or oxitropium (called anticholinergics).
You may need to take different amounts of your medicines or you may need to take different medicines. Your doctor and pharmacist have more information.
If you have not told your doctor about any of these things, tell him/ her before you start using BRETARIS GENUAIR.
How to use BRETARIS GENUAIR
Follow all directions given to you by your doctor and pharmacist carefully.
These directions may differ from the information contained in the leaflet.
If you do not understand the instructions, ask your doctor or pharmacist for help.
How much to use
The recommended dose is one inhalation twice a day in the morning and night.
The effects of BRETARIS GENUAIR last for 12 hours; therefore, you should try to use your BRETARIS GENUAIR inhaler at the same time every morning and at night. This ensures that there is always enough medicine in your body to help you breathe more easily throughout the day and night. It will also help you to remember to use it.
How to use
See instructions at the end of this leaflet or refer to booklet contained within the carton for instructions on how to use the BRETARIS GENUAIR inhaler.
If you are not sure of how to use BRETARIS GENUAIR, ask your doctor or pharmacist.
How long to use
Continue to use this medicine for as long as your doctor tells you to.
If it helps your breathing problems, your doctor may want you to keep using it for a long time.
This medicine helps to control your condition but it does not cure it.
If you want to stop treatment, first talk to your doctor, as your symptoms may worsen.
If you forget to use it
If you forget to have a dose of BRETARIS, inhale the dose as soon as you remember. However, if it is nearly time for your next dose, skip the missed dose.
Do not take a double dose to make up for the one that you missed.
This may increase the chance of you getting an unwanted side effect.
If you have trouble remembering when to use your medicine, ask your pharmacist for some hints.
If you use too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice, or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have used too much BRETARIS. Do this even if there are no signs of discomfort or poisoning.
While you are using BRETARIS GENUAIR
Things you must do
Use this medicine exactly as your doctor has prescribed. Try not to miss any doses and use it even if you feel well.
If you do not follow your doctor’s instructions, you may not get relief from your breathing problems or you may have unwanted side effects.
If you find that the usual dose of BRETARIS is not giving as much relief as before, or does not last as long as usual, contact your doctor so that your condition can be checked.
This is important to ensure your COPD is controlled properly.
If you become pregnant while using this medicine, tell your doctor.
Your doctor can discuss with you the risks of using it while you are pregnant.
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are using BRETARIS GENUAIR.
Tell any other doctors, dentists, and pharmacists who treat you that you are using this medicine.
Things you must not do
Do not exceed the recommended daily dose – it will not help you to do this.
Do not take any other medicines for your breathing problems without checking with your doctor.
Do not give this medicine to anyone else, even if their condition seems similar to yours.
Do not use it to treat any other complaints unless your doctor tells you to.
Things to be careful of
Be careful driving, operating machinery or doing jobs that require you to be alert until you know how BRETARIS GENUAIR affects you.
This medicine may cause headache, dizziness or blurred vision. If you are affected by either of these side effects do not drive or use machinery until the headache has cleared and your vision has returned to normal.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are using BRETARIS GENUAIR.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
Do not be alarmed by these lists of possible side effects. You may not experience any of them. Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
headache
inflammation of the sinuses (sinusitis)
runny nose or sneezing (rhinitis)
common cold or flu symptoms
cough
diarrhoea, vomiting, nausea or abdominal discomfort
dry mouth
changes in voice
fungal infection in the mouth (candidiasis)
dizziness
toothache or infection in the tissues at the base of a tooth (tooth abscess)
inflammation of the mouth (stomatitis)
blurred vision
fall
heart failure, diabetes, arthritis
itchy rash.
Tell your doctor as soon as possible if you notice any of the following:
palpitations (feeling your heartbeat is unusually fast or irregular)
difficulty passing urine (urinary retention).
Tell your doctor immediately or go to Accident and Emergency at your nearest hospital if any of the following happen:
you develop signs of an allergic reaction such as swelling of the face, lips, tongue, throat or other part of the body; severe dizziness or fainting, faster heart rate, redness, itching or rash on the skin
if you get tightness of the chest, coughing, wheezing or breathlessness immediately after using the medicine. These may be signs of a condition called bronchospasm.
Tell your doctor or pharmacist if you notice anything else that is making you feel unwell.
After using BRETARIS GENUAIR
Storage
Keep the BRETARIS GENUAIR inhaler protected in the sealed pouch until you need to start using it.
The inhaler must be used within 90 days of opening the pouch.
Keep your inhaler in a cool dry place where the temperature stays below 30°C.
Do not store BRETARIS GENUAIR or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car.
Heat and dampness can destroy some medicines.
Keep it where children cannot reach it.
A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop using the inhaler or the expiry date has passed, ask your pharmacist how to dispose of it properly.
Product description
What it looks like
BRETARIS is a white or almost white powder.
The BRETARIS GENUAIR inhaler device is white coloured with an integral dose indicator and a green dosage button. The mouthpiece is covered with a removable green protective cap. It is supplied in a sealed pouch.
Ingredients
Active Ingredient
Each delivered dose contains 375 micrograms aclidinium bromide (equivalent to 322 micrograms of aclidinium).
Inactive Ingredient
BRETARIS also contains lactose monohydrate.
Supplier
BRETARIS GENUAIR is supplied in Australia by:
A. Menarini Australia Pty Ltd
Level 8, 67 Albert Avenue
Chatswood NSW 2067
Medical Information: 1800 644 542
® = Registered Trademarks of Almirall, S.A. and AstraZeneca AB
Australian Registration Number(s): AUST R 206071
This leaflet was revised in February 2020.
For the most up to date version of this document, please go to www.menarini.com.au/cmi
[vA04-0]
Instructions for Use
Getting Started
Before using the BRETARIS GENUAIR inhaler, please read the full instructions.
You should become familiar with the parts of your inhaler (see image 1).
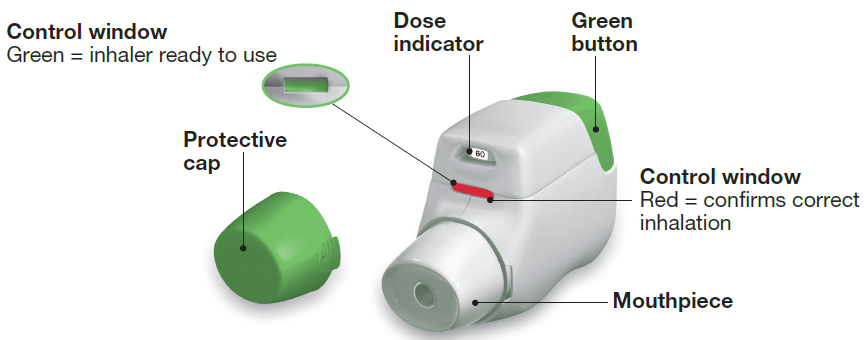
IMAGE 1
Before Use
Before first use, tear the sealed pouch along the notch and remove the inhaler.
Do not press the green button until you are ready to take a dose.
When you are about to take your dose of medicine, remove the protective cap by LIGHTLY SQUEEZING THE ARROWS marked on each side and pulling outwards (see image 2).
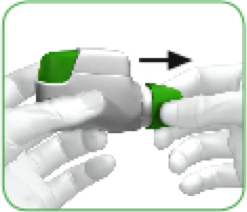
IMAGE 2
Step 1: Activate your inhaler
1.1 Look to see that nothing is blocking the mouthpiece (see image 3).
1.2 Look at the coloured control window and it should be red (see image 3).
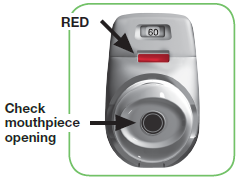
IMAGE 3
1.3 Hold the inhaler horizontally with the mouthpiece towards you and the green button facing straight up (see image 4).
Make sure the green button is on top. DO NOT TILT.
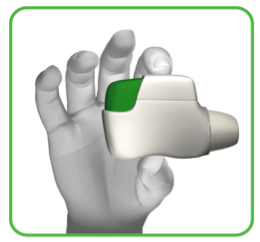
IMAGE 4
1.4 Press the green button all the way down to load your dose (see image 5). When you press the button all the way down, the control window changes from red to green.
1.5 Release the green button (see image 6).
Make sure you release the green button so the inhaler can work correctly.
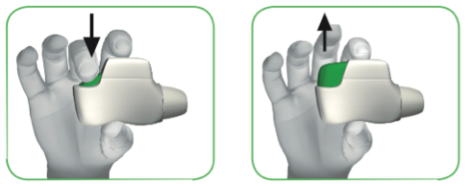
IMAGE 5 IMAGE 6
Stop and Check: Make sure your dose is ready for inhalation
1.6 Make sure the coloured control window has changed to GREEN (see image 7).
The green control window confirms that your medicine is ready for inhalation.
IF THE COLOURED CONTROL WINDOW STAYS RED AFTER PRESSING THE GREEN BUTTON ALL THE WAY DOWN, GO BACK TO ‘STEP 1 ACTIVATE YOUR INHALER’ AND REPEAT STEPS 1.1 TO 1.6.
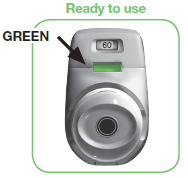
IMAGE 7
Step 2: Inhale your dose
Read steps 2.1 to 2.7 fully before use. DO NOT HOLD THE GREEN BUTTON DOWN WHILE INHALING. Do not tilt.
2.1 Before bringing the inhaler to your mouth, breathe out completely away from the mouthpiece. Do not breathe out into the mouthpiece of the inhaler.
2.2 Hold your head upright and close your lips tightly around the mouthpiece of the inhaler.
2.3 Breathe in STRONGLY and DEEPLY through your mouth (see image 8). This strong, deep breath pulls the medicine through the inhaler into your lungs.
While you breathe in you will hear a “CLICK” which signals that you are inhaling correctly. Keep breathing in for as long as possible even after you have heard the “CLICK” to be sure you get the full dose.
ATTENTION: DO NOT HOLD THE GREEN BUTTON DOWN WHILE YOU ARE INHALING. DO NOT TILT.
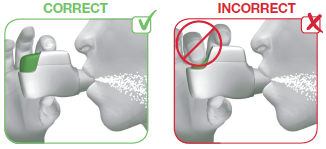
IMAGE 8
2.4 Remove the inhaler from your mouth.
2.5 Hold your breath for as long as is comfortable.
2.6 Breathe out slowly through your nose, away from the mouthpiece.
Some patients may experience a grainy sensation in their mouth, or a slightly sweet or bitter taste when inhaling the medicine. Do not take an extra dose if you do not taste or feel anything after inhaling.
Stop and Check: Make sure you have inhaled correctly
2.7 Make sure the control window is now RED (see image 9). This confirms that you have inhaled your full dose correctly.
IF THE COLOURED CONTROL WINDOW IS STILL GREEN, THIS MEANS YOU HAVE NOT INHALED YOUR FULL DOSE CORRECTLY. GO BACK TO ‘STEP 2 INHALE YOUR DOSE’ AND REPEAT STEPS 2.1 TO 2.7.
If the control window still does not change to RED, you may have forgotten to release the green button before inhaling or may not have inhaled correctly. If that happens, try again.
Make sure you have RELEASED the green button and take a STRONG DEEP breath in through the mouthpiece.
Note: If you are unable to inhale correctly after several attempts, consult your doctor.
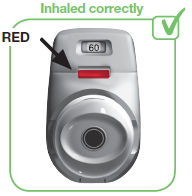
IMAGE 9
Once the control window has turned red, replace the protective cap by pressing it back onto the mouthpiece (see image 10). This will prevent moisture from getting inside the mouthpiece of your inhaler.
IMAGE 10
Additional Information
What should you do if you accidentally prepared a dose?
Store your inhaler with the protective cap in place until it is time to inhale your dose, then remove the cap and start at Step 1.6.
How should you clean the inhaler?
You DO NOT NEED to clean your inhaler. However, if you wish to clean it you should do so by wiping the outside of the mouthpiece with a dry tissue or paper towel.
NEVER use water to clean the inhaler, as this may damage your medicine.
How does the dose indicator work?
The inhaler is equipped with a dose indicator to show you approximately how many doses are left in the inhaler.
On first use, every inhaler contains at least 60 doses, or at least 30 doses, depending on the pack size.
Each time you load a dose by pressing the green button all the way down, the dose indicator moves down slowly displaying intervals of 10 (60, 50, 40, 30, 20, 10, 0) (see image 11).
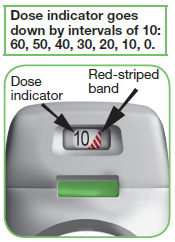
IMAGE 11
When should you get a new inhaler?
You should get a new inhaler:
When a RED STRIPED BAND appears in the dose indicator (see image 11); this means you are nearing your last dose, or
If your inhaler appears to be damaged or if you lose the cap, or
If your inhaler is empty (see ‘How do you know that your inhaler is empty?’).
How do you know that your inhaler is empty?
When 0 (zero) appears in the middle of the dose indicator, you should continue using any doses remaining in the inhaler.
When the last dose has been prepared for inhalation, the green button will not return to its full upper position, but will be locked in a middle position (see image 12). Even though the green button is locked, your last dose may still be inhaled. After that, the inhaler cannot be used again and you should start using a new BRETARIS GENUAIR inhaler.

IMAGE 12











