
Understanding how the brain works is a paramount goal of medical science. But with its billions of tightly packed, intermingled neurons, the human brain is dauntingly difficult to visualize and map, which can provide the route to therapies for long-intractable disorders.
In a major advance published next week in Nature, scientists for the first time report the structure of a fundamental type of tissue organization in brains, called neuropil, as well as the developmental pathways that lead to neuropil assembly in the roundworm C. elegans. This multidisciplinary study was a collaboration between five laboratories, including scientists at the Marine Biological Laboratory (MBL), Woods Hole, which hosted much of the collaboration.
“Neuropil is a tissue-level organization seen in many different types of brains, from worms to humans,” says senior author and MBL Fellow Daniel Colón-Ramos of Yale School of Medicine. “When things are that conserved in nature, they are important.”
“But trying to understand neuropil structure and function is very challenging. It’s like looking at a spaghetti bowl,” Colón-Ramos says. “Hundreds of neurons are on top of each other, touching each other, making thousands of choices as they intermingle through different sections of the animal’s brain. How can you describe neuropil organization in a way that’s comprehensible? That is one of the contributions of this paper.”
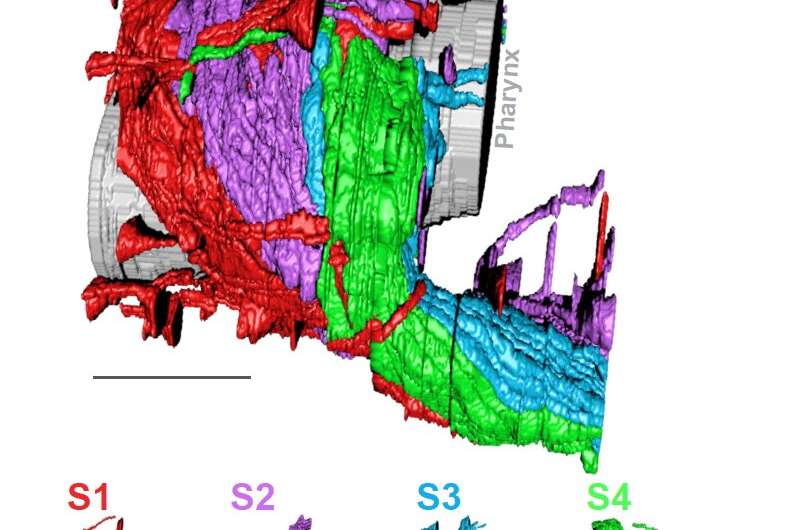
The authors focused on the neuropil in the C. elegans nerve ring, a tangled bundle of 181 neurons that serves as the worm’s central processing unit. Through an innovative melding of network analysis and imaging strategies, they revealed that the nerve ring is organized into four layers, or strata. These strata, they showed, contain distinct domains for processing sensory information and motor behaviors. They were able to map the worm’s sensory organs and muscle quadrants onto the relevant strata.
The team also discovered unique neurons that integrate information across strata and build a type of “cage” around the layers. Finally, they showed how the layered structure of the neuropil emerges in the developing worm embryo, using high-resolution light-sheet microscopy developed by MBL Fellow Hari Shroff of the National Institute of Biomedical Imaging and Bioengineering, and MBL Investigator Abhishek Kumar.
“This is a paradigm shift where we combined two fields—computational biology and developmental biology—that don’t often go together,” says first author Mark Moyle, associate research scientist in neuroscience at Yale School of Medicine. “We showed that by using computational approaches, we could understand the neuropil structure, and we could then use that knowledge to identify the developmental processes leading to the correct assembly of that structure.”
This approach can serve as a blueprint for understanding neuropil organization in other animal brains, the authors state.
From Buildings to Boroughs to New York City
C. elegans has the best understood nervous system of all animals. More than 30 years ago, John White, Sydney Brenner, and colleagues published the worm’s “connectome”—a wiring diagram of its 302 neurons and the ~7,000 synaptic connections between them. Since that pioneering study, nearly every neuron in C. elegans has been characterized: its shape, functional category, the neural circuits it participates in, and its developmental cell lineage.
What was missing, though, was a picture of how these cells and circuits integrate in space and over time.
Colón-Ramos and team analyzed published data on all the membrane contacts between the 181 neurons in the nerve ring. They then applied novel network analyses to group cells into “neighborhoods” based on their contact profiles—similar in principle to algorithms that Facebook uses to suggest friends based on people’s common contacts. This revealed the neuropil’s layered structure and enabled the team to understand cell-cell interactions in the context of functional circuits, and functional circuits in the context of higher-order neuropil structure (See video 1).
“All of a sudden, when you see the architecture, you realize that all this knowledge that was out there about the animal’s behaviors has a home in the structure of the brain,” Colón-Ramos says.
“By analogy, rather than just having knowledge of the East Side of New York and the West Side, Brooklyn and Queens, suddenly you see how the city fits together and you understand the relationships between the neighborhoods.”
“So now we could see, ‘OK, this is why these behaviors are reflex-like, because they are direct circuits that go into the muscles. And this is how they integrate with other parts of the motor program.’ Having the structure allows you to generate new models regarding how information is being processed and parceled out to lead to behaviors,” Colón-Ramos says.
Reconstructing the Birth and Development of the Nerve Ring
The brain is a product of development, starting with one embryonic cell division and ending with a complete organ. “An order emerges through time. So our next question was, how can you instruct the formation of a layered structure? How are all these decisions simultaneously occurring in hundreds of cells, but resulting in organized layers? How are the decisions coordinated through time and space?” Colón-Ramos says.
“Layered structures are a fundamental unit of brain organization—the retina is a layer, and the cortex is a layer. If we could understand it for the worm, it would allow us to create models that might help us understand the development of layers in other vertebrate organs, like the eye,” Colón-Ramos says.
This part of the research began in 2014 when Colón-Ramos and Moyle began collaborating with microscope developers Shroff and Kumar at the MBL. “We started by building a microscope (the diSPIM) that let us look at the embryo with better spatial and temporal resolution than the tools of the time,” Shroff says.
They then identified every cell in the C. elegans embryo using lineaging approaches developed by co-author Zhirong Bao of Sloan Kettering Institute (these findings are catalogued at WormGUIDES.org). “This was a painful process, but very important to do,” Shroff says.
After years of sharing a lab at the MBL, numerous adjustments to the diSPIM system, integrations with other critically important technology, and plenty of frustration, the collaborators succeeded in resolving the developmental sequence of the C. elegans neuropil and revealing principles that guide its stratified organization (see video 2).
Source: Read Full Article