
Dilated cardiomyopathy (DCM) is a leading cause of heart failure, affecting 1 in 250 people. DCM is characterized by an increase in the left ventricle of the heart, the main pumping chamber. The stretched heart muscle is then unable to pump blood as well, which can lead to irregular heartbeats, heart valve problems, and, ultimately, heart failure. As the leading cause of heart failure, DCM is the most common reason for heart transplant, only offered at end-stage heart failure when all other treatment options and lifestyle changes have failed. Despite years of work to improve patient survival after transplantation, there is still only a 10-year survival rate of 50%. DCM patient health, therefore, would be significantly improved with better treatment options prior to the need for a heart transplant.
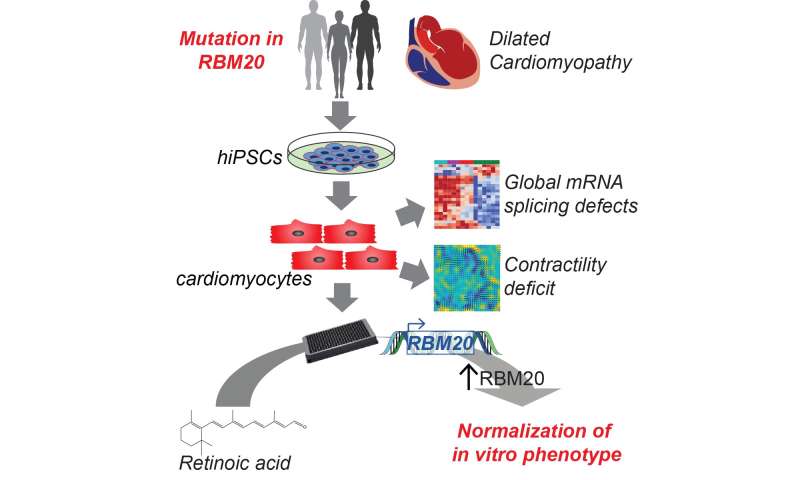
Many instances of DCM are caused by inherited changes (mutations) in a person’s DNA. Yet, despite detailed knowledge of the mutations, there are no mutation specific therapies. The relatively recent ability to make cardiomyocytes from induced pluripotent stem cells (iPSC-CMs) has made it possible to model and understand genetic heart disease as well as search for treatments. A group of researchers, led by first-authors Francesca Briganti, Ph.D., and Han Sun, Ph.D., and senior authors Mark Mercola, Ph.D., and Lars Steinmetz, Ph.D., in collaboration with Ioannis Karakikes, Ph.D., also at Stanford, used a combination of iPSC-CMs and genome editing to identify a new mutation causing DCM and in doing so discovered a potential treatment option.
In their paper, recently published in Cell Reports, the researchers had the unique opportunity to study a single family with inherited DCM to understand the cause of their disease. As an inherited disease, information on the genome, or complete set of DNA and genes, can provide important information on the mutation causing the disease. Each gene contains instructions for making a specific protein needed by the cell, and the gene is passed from parent to child, including changes in the protein that result in disease such as inherited DCM. In this case, the team compared the genomic pieces that make up the proteins (the exome). By studying a family, they were able to look for differences in the exomes of someone who had passed away with a diagnosis of DCM, had been diagnosed with DCM, or was unaffected by DCM. This comparison allowed them to find a single mutation (P633L) in a protein, RBM20, that was disease causing. Although the mutation was unknown, changes in RBM20 are known to cause a severe form of inherited DCM that is often associated with early onset of end-stage heart failure. It is also thought that normal RBM20 function is lost when only one copy of the gene is changed (mutated) and the other is normal; this is called haploinsufficiency.
The iPSC and especially iPSC-CM platform is a powerful tool for studying specific cardiovascular diseases such as inherited DCM. Researchers were able to use genome editing, the process of making specific changes to the DNA of a cell, to introduce the RBM20 mutation (P633L) into iPSC-CMs. This gave them the chance to understand how the mutation was causing DCM.
Source: Read Full Article